by CongCong Li, Frank van Steenbergen, Salma Enan, Meghna Mukerjee

Detergents matter
Detergents matter. Detergents matter a lot. There is a seesaw difference between low-quality and high-quality detergents. This is particularly important when washing is done by hand, as is the case for millions and millions of rural women. Detergent quality determines the effort and time that goes into washing, how effectively the pathogen cycles are broken, whether irritation and allergic reactions are common, and how water quality is affected.
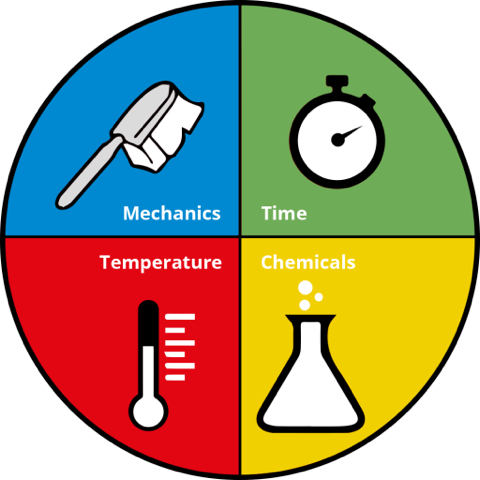
How do detergents work? The Sinner’s Cycle[1] is a shorthand of how laundry practice works. In washing clothes, there are four factors influencing the outcome: agitation/mechanics, temperature, time, and chemical/detergent. The four factors are somehow interchangeable. For example, when using cold water to wash clothes, increased agitation or a more effective detergent may be required; soaking dirty laundry for a longer period can improve the washing results.
[1] The “Sinner’s Circle” is named after Dr. Sinner, also named the cleaning circle. It outlines the mechanisms involved in cleaning, including four key factors: time, mechanical force, chemistry, and temperature. These factors are interdependent and form a closed system. When one or two factors are increased, the others are automatically reduced, and vice versa.
Detergents are an essential component of laundry. It is a cleaning product designed for washing clothes and textiles. Detergent products on the market can be categorized into powder detergents, liquid detergents, detergent bars, detergent pods, etc. The primary purpose is to remove dirt, stains, sweat, oils, pathogens, and other contaminants from fabrics, leaving them clean, fresh, and often pleasantly scented. Laundry detergents are also designed to work effectively in different water temperatures and cleaning methods, whether in warm or cold water, using a washing machine, or washing by hand. This blog will provide a basic overview of how detergents work.
Exit phosphate-based detergents
Two types of detergents can be found on the market: phosphate-based and surfactant-based detergents. Phosphate is a common additive in laundry detergents because it could enhance detergents’ effectiveness by binding with calcium and magnesium ions. Yet, Laundry detergents containing phosphates can harm users’ health by irritating the skin and causing hives(Insinc, n.d.). Additionally, phosphates bring low toxicity to the environment. With laundry wastewater, Phosphates will eventually make their way to natural bodies of water. This will cause nutrient pollution and feed algae, leading to eutrophication and harmful algal blooms(Bajpai, 2007). Scientific experiments indicate that even concentrations as low as two ppm can double the chemical absorption in fish, leading to environmental damage (Bajpai, 2007). This slow poisoning process is also one of the main reasons we have shifted from drinking tap water to bottled spring water (Bajpai, 2007).
In response to environmental and health concerns, the government has implemented regulations on the use of phosphates in detergents. For instance, the US and the EU implemented regulations on the use of phosphates in consumer laundry products in 2010 and 2013, respectively. Looking at the Asian detergent market, governments in some countries have also enacted stringent “ phosphate-free detergent regulations” to reduce the use of phosphates in households. For example, China implemented the Hazard Safety Management Regulations in 2002. The same was addressed by the Environment Protection Act of 1986 in India and many subsequent regulations. However, evidence shows that phosphate levels used in detergent products often still exceed regulatory standards in countries such as India, Indonesia, and the Philippines (Stellar, 2024). As a transitioning country, the Chinese detergent market has encountered several challenges: increased production costs, supply chain and sourcing difficulties, and consumer resistance to price hikes(Stellar, 2024). Therefore, stakeholders such as detergent manufacturers, consumers, and development agencies still have much work to do. Luckily, with increasing environmental awareness among consumers and attention from relevant stakeholders, there is a growing focus on sustainable innovations in the Asian detergent industry. For example, Detergent firms in the Asia Pacific are shifting towards more eco-friendly practices. This includes formulating detergents with reduced chemical footprints. Additionally, advancements in wastewater treatment technologies are being explored to mitigate the impact of phosphate pollution.
Enter surfactant-based laundry detergents
To mitigate the environmental concern associated with phosphates, the transition towards surfactant-based laundry has been happening in many parts of the world. The fundamental mechanisms of surfactant detergent are similar across products, incorporating components such as surfactants, enzymes, builders, and other additives.
Surfactant is a key ingredient in detergent formulation, characterized by a unique molecular structure that allows them to interact with contrasting surfaces like oil and water. By lowering the surface tension of water, surfactants help penetrate fabrics more efficiently, facilitating the removal of dirt, grease, and oils effectively. They also lift dirt and grease from fabrics while preventing them from reattaching to the clothing. A detergent typically has multiple types of surfactants. These surfactants vary in their capacity to remove different types of soil, their effectiveness on various fabrics, and their performance in different water hardness conditions. Common surfactants such as Sodium Lauryl Sulfate (SLS) and Alkyl Benzene Sulfonate (LABSA) are essential for foaming and cleaning. Secondary surfactants like Cocamide DEA (CDEA) and Cocamidopropyl Betaine (CAPB) enhance the detergent’s cleaning power and foam stability.
Surfactants could lose efficiency in hard water due to excess calcium (Ca²⁺) and magnesium (Mg²⁺) ions, which interfere with their action (Frydendall, 2024). To solve it, Builders become essential to enhance the cleaning efficiency of the surfactant. Builders are designed to soften water by binding hard water minerals and preventing water hardness ions. They help surfactants concentrate on removing soil from fabrics and increase their efficiency. Additionally, builders provide a desirable level of alkalinity to aid in the cleaning process and disperse and suspend soiled particles so they cannot redeposit themselves on the clothing.

Enzymes are also crucial for detergent performance, as they help break down food particles on clothing by catalyzing or accelerating the decomposition process. Hydrolases are the most widely used detergent enzymes, which remove protein, lipid, and polysaccharide soils (Novozymes,n.d.). Currently, the enzymes used in detergents are categorized as shown in Figure 2. Researchers are also working to expand the variety of enzymes used in detergents.
| Four classes of enzymes are generally used in detergent | |
| Proteases | The most commonly used enzymes in the detergent industry; remove protein stains such as grass, blood, egg and human sweat which tend to adhere strongly to textile fibers |
| Amylases | Used to remove residues of starch-based foods like potatoes, spaghetti, custards, gravies and chocolate. |
| Lipases | Decompose fatty material. Lipase is capable of removing fatty stains such as fats, butter, salad oil, sauces and the tough stains on collars and cuffs. |
| Cellulases | Modify the structure of cellulose fiber on cotton and cotton blends. When it is added to a detergent, it results in; color brightening, softening and soil removal. |
Figure 2: based on Vanjare & Waghmode (2020).
Other ingredients include bleaching agents, chelating agents, fragrances, colorants, preservatives, and optical brighteners. By removing color from stains and soils, the bleaching agents could whiten and brighten fabrics. They also enhance the antimicrobial activity of the laundering process. The most commonly used bleaching agents are hydrogen peroxide and sodium perborate. Chelating agents could prevent the interference of hard water minerals, improving laundry performance. Common chelating agents include Ethylenediaminetetraacetic Acid (EDTA), sodium gluconate, and Diethylenetriaminepentaacetic acid (DTPA). Fragrances, colorants, and preservatives can enhance the presentation, appeal, and shelf life of laundry detergent liquids. Fragrances add a pleasant scent, while colorants provide visual appeal. Preservatives prevent microbial growth and extend the product’s shelf life. Lastly, optical brighteners can enhance color brightness by absorbing ultraviolet light and emitting visible light. This creates the illusion of fabrics appearing brighter and cleaner.
What’s worth noting is that the pH level of a detergent is a critical factor in its cleaning performance. Most liquid detergents are formulated with an alkaline pH ranging from 7 to 11, which aids in soil removal and enhances the effectiveness of surfactants and enzymes (Stellar, 2024). Buffering agents such as sodium carbonate and sodium citrate are used to maintain a stable pH throughout the detergent’s lifecycle, ensuring consistent cleaning performance. The innovations in surfactant and enzyme technology have revolutionized the detergent industry, leading to more effective, efficient, and environmentally friendly cleaning solutions.
By understanding each component’s role in the detergent formula, the industry can design innovative, high-performance laundry detergents that meet consumer needs and adhere to regulatory standards. This is particularly important for the often forgotten yet large bottom-of-the-pyramid market – of poor people still relying on handwashing– with limited agitation, the use of relatively cold water, and sometimes very scarce good quality water. In addition, these people face specific challenges such as cold weather that makes drying clothes difficult, heavily soiled garments from manual labor, and thick items like blankets and quilts that can trap numerous pathogens. There is a need and a large market opportunity to develop and market detergent formula targeted at these important and widespread challenges.
(This blog is prepared as part of the LIFT-Us Initiative.)
Reference List
Barrett, B., Brunelle, E., & Podas, M.(1973). Detergents, phosphates, and environmental control. Journal of Food Protection, 36(7), 368-370.
Bajpai, D. (2007). Laundry detergents: an overview. Journal of oleo science, 56(7), 327-340.
Stellar Market Research. (2024). Asian Pacific laundry detergent market industry analysis and forecast (2024-2030). https://www.stellarmr.com/report/Laundry-Detergent-Market/372
European Union. (2011, December 14). EP supports ban of phosphates in consumer detergents. https://ec.europa.eu/commission/presscorner/detail/en/ip_11_1542
Insinc.(n.d.).Why choose phosphate-free detergents? . https://www.insinc.co.nz/blog/phosphate-free-detergents?srsltid=AfmBOooy1-piemHRO-TDRk7kaVL0T2WIhfzA9nPPBJnURZDW2K0seoe8
Frydendall, E. (2024, April 16). How laundry detergent works. https://home.howstuffworks.com/laundry-detergent.htm
Kumar, G., Malik, K., & Tiwari, P. (1998). Novel enzyme-based detergents: an Indian perspective. Current Science, 75(12), 1312-1318.
Novozymes(n.d.). A beginner’s guide to enzymes in detergents https://biosolutions.novozymes.com/en/dish/insights/article/beginners-guide-enzymes-detergents
Vanjare, J., & Waghmode, S. (2020). Lipase enzyme based green chemistry detergents for the cleaning industry.
Xie, J. (2023, May 27). Mastering Laundry Detergent Liquid Formulation: A Comprehensive Guide for Industry Insiders. https://yeserchem.com/mastering-laundry-detergent-liquid-formulation-a-comprehensive-guide-for-industry-insiders/



